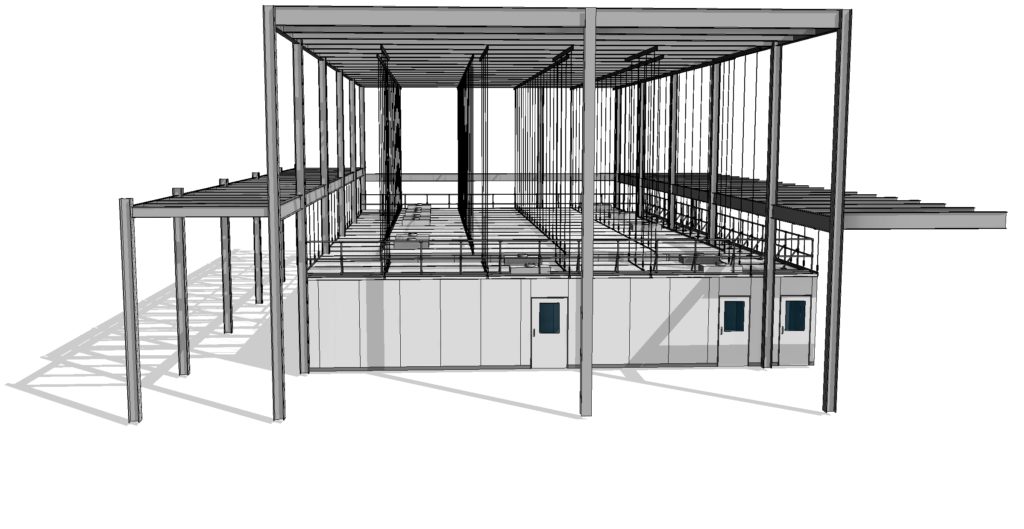
ABOVE: The AESystem can be assembled anywhere. Box in box installation.
Since 2000, the AES team has been working with companies in their development of autologous and allogeneic cell and tumor processing technologies. As this market segment has evolved (and continues to evolve), AES has continued to work with clients to develop modular cleanroom technology that drives efficient, safe and compliant process operations. Processing methods today are improving, yet with open process connections and the utilization of a variety of benchtop process systems, maintaining control, cleanliness and segregation in these Grade A, B, C and D process suites is of paramount importance. AES understands this importance, and has successfully developed many projects including those listed below. We are very proud to have helped these companies bring vital new and novel therapies to market, and we are dedicated to continued support in this evolving and growing market segment.
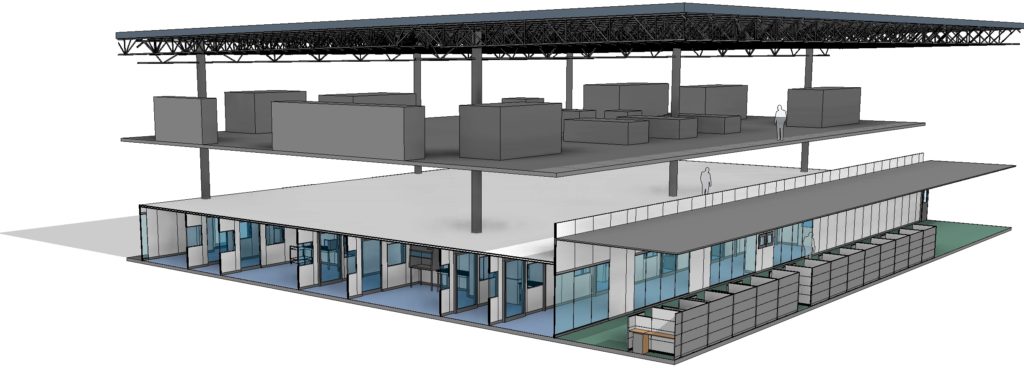
ABOVE: 3 representation of classified space containing multiple processing suites.
Cell & Gene Therapy Client List
• Astellas • Erytech • University of MD • Lonza Biologics • Novartis • Cook Regentec
• Stemcyte • Progenitor Cell Therapy • Dendreon • UPenn • Brammer • Pfizer • Kite
• Aldevron • Atara • Advaxis • Duke • HCATS • Macrogenics • Rocket Pharma • Novartis
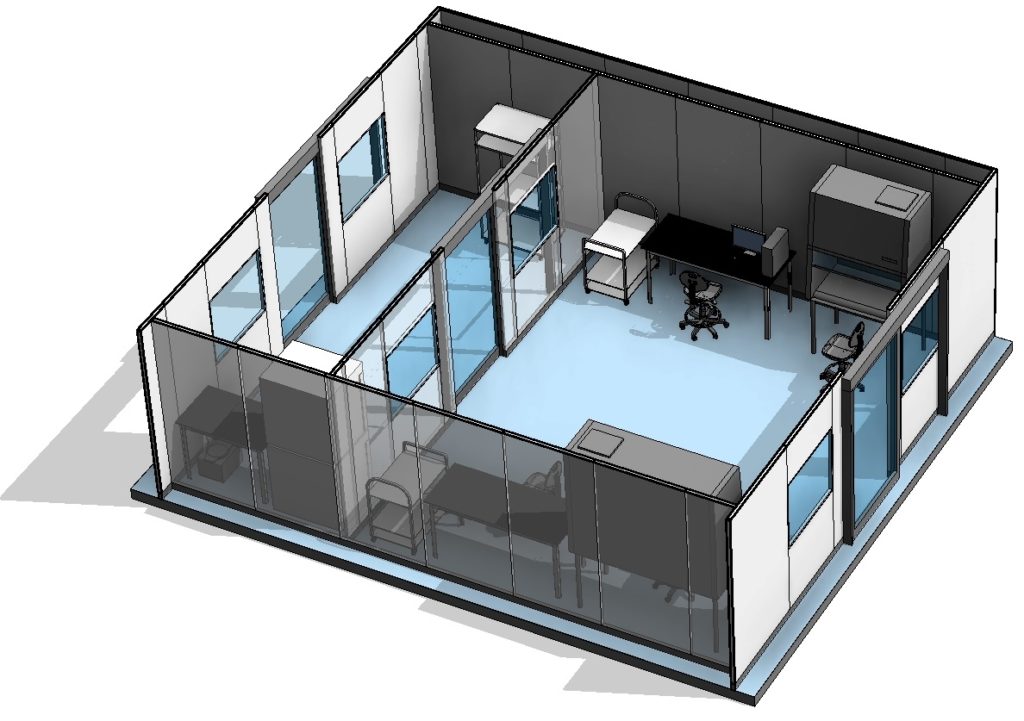
ABOVE: 3d image of a processing suite.
